The Gan lab officially launched on July 1st, 2025 at the Aging + Cardiovascular Discovery Center (ACDC) and the Department of Cardiovascular Sciences at Temple University, Lewis Katz School of Medicine.
Synergizing with the robust cardiovascular research infrastructure at Temple University, we employ advanced RNA biology and condensate biology techniques to investigate novel posttranscriptional and transcriptional regulatory mechanisms within the cardiovascular system.
Research
Cardiovascular disease is the leading cause of death in the United States and worldwide. Built upon the PI’s research findings and expertise, the Gan lab focuses on the posttranscriptional and transcriptional regulation in the cardiovascular system. The overarching goal of our lab is to investigate new posttranscriptional regulators (RNA binding proteins) and novel transcriptional condensate-based regulatory mechanisms in various aspects of cardiovascular biology. The ultimate of goal is to translate our discoveries into treatments for cardiovascular diseases.
RNA binding proteins in cardiovascular physiology and pathology (details ↓)
RNA posttranscriptional processing has been increasingly recognized as a key process to regulate cardiac gene expression and function. RNA binding proteins (RBPs) modulate various RNA processings, such as alternative splicing, transportation, translation and stabilization. In mammalian heart, more than 400 RBPs are specifically expressed, building an intricate posttranscriptional regulatory network together with hundreds of other ubiquitous or undiscovered RBPs. Emerging evidence showing that cardiac RBPs play crucial roles in heart development and diseases, while the cardiac function of vast majority of RBPs remains unclear. In recent years, technical advances in studying protein-RNA interactions and RNA biology largely promote the investigation of cardiac RBPs. In many fields, modulation of RBPs and relevant RNA processing have emerged as an effective strategy to prevent or cure diseases. Integrating cutting-edge techniques in RNA biology research and a strong cardiovascular research infrastructure, we aim to develop an ambitious and impactful cardiovascular RNA binding proteome research program to uncover new posttranscriptional regulatory mechanisms and find novel therapeutic strategies for cardiovascular diseases.
Relevant publications:
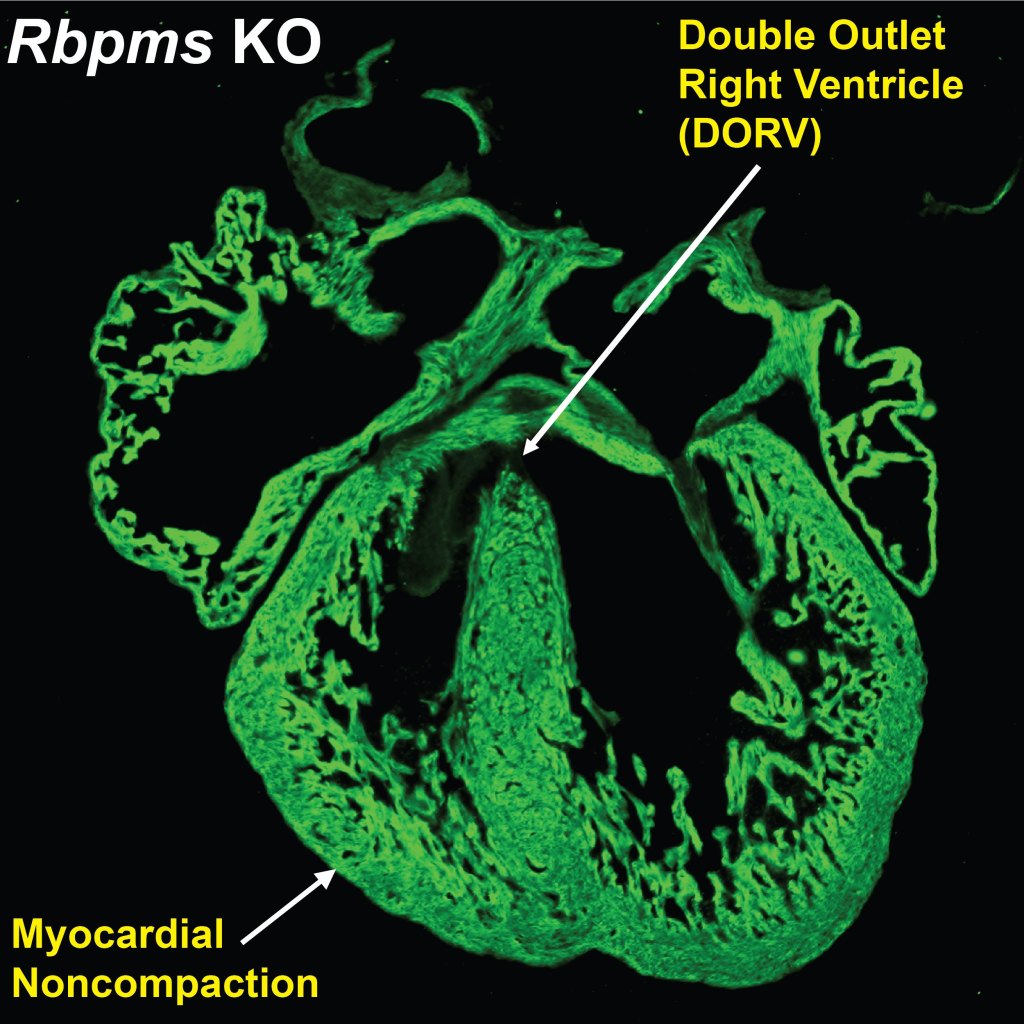
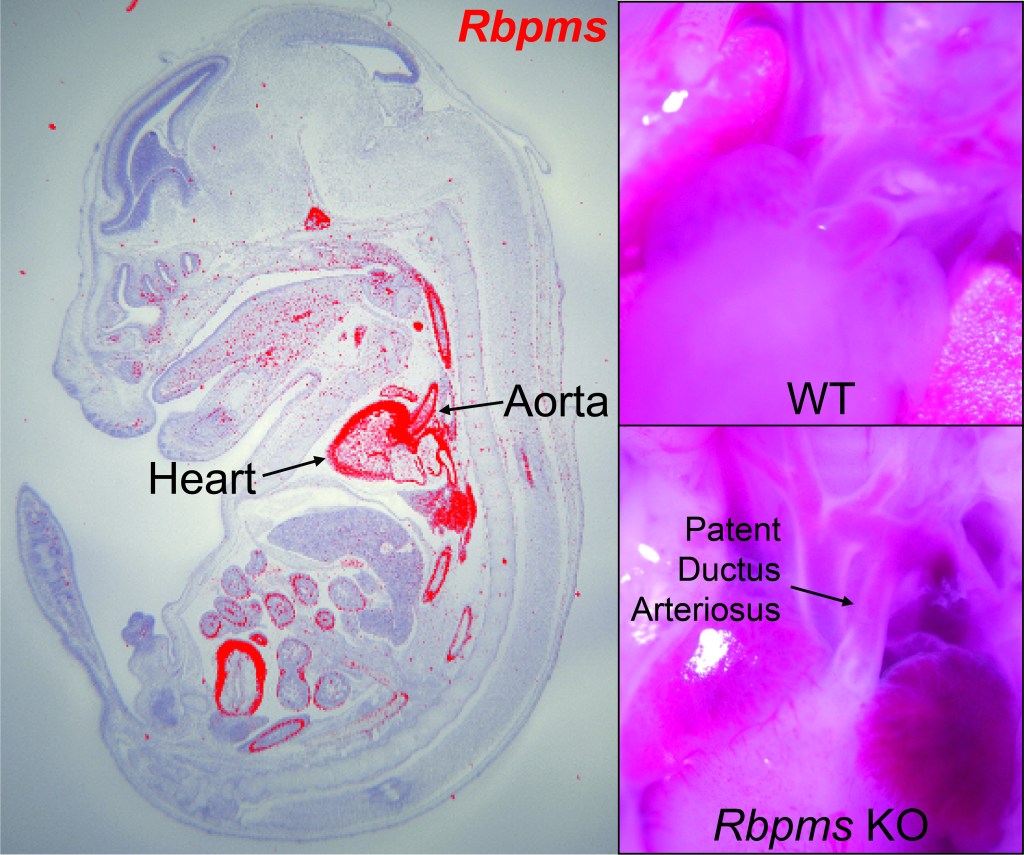
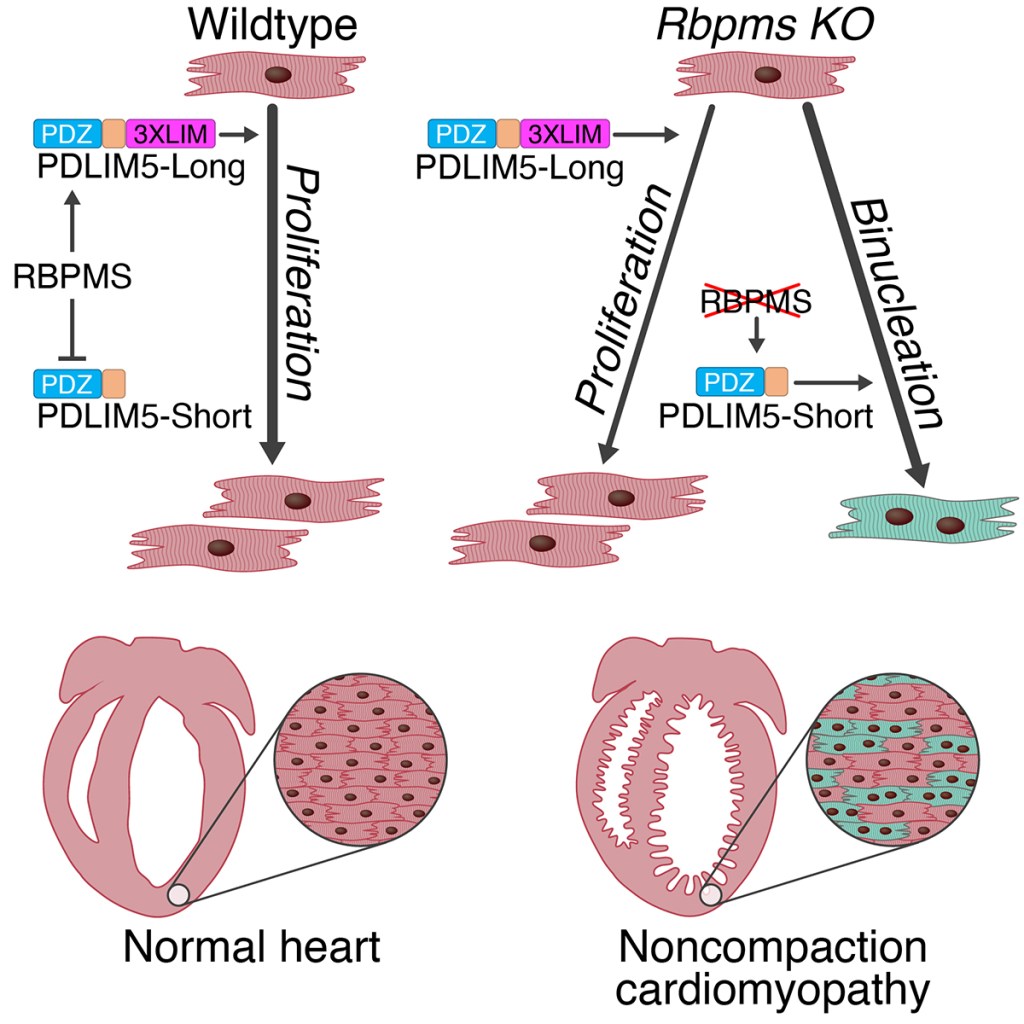
- Gan P, et al. RBPMS is an RNA-Binding Protein that Mediates Cardiomyocyte Binucleation and Cardiovascular Development. Developmental Cell. 2022 Apr 25;57(8):959-973.e7.
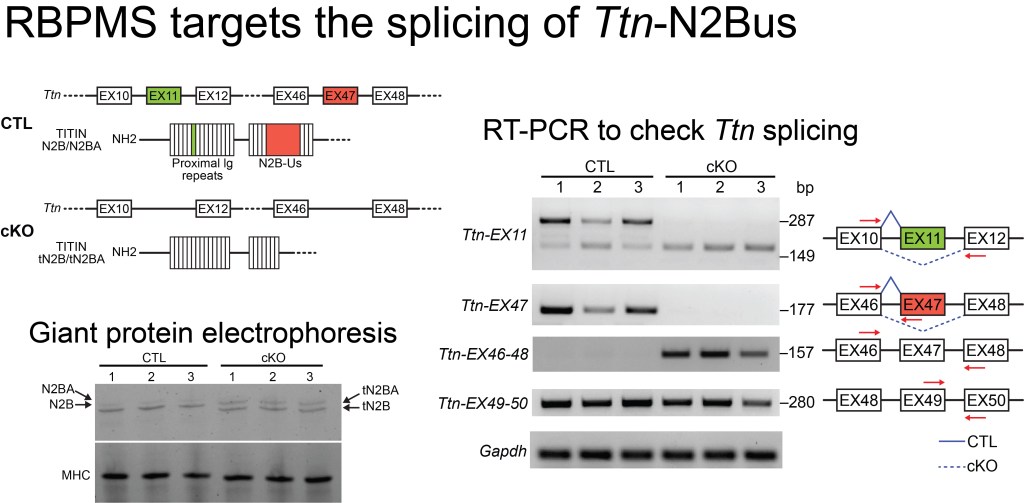
- Gan P, et al. RBPMS Regulates Cardiomyocyte Contraction and Cardiac Function through RNA Alternative Splicing. Cardiovascular Research. 2024 Feb 27;120(1):56-68.
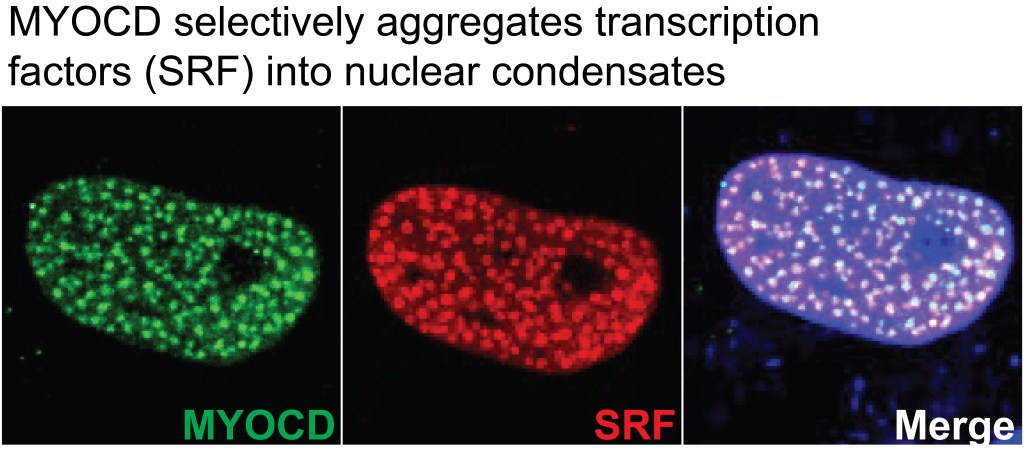
Transcriptional condensates in cardiovascular development and disease (details ↓)
Precise and decisive activation of new gene programs is a crucial step in cell state transition. Recent studies have shown that general transcriptional coactivators activate genes in part through the formation of biomolecular condensates, dynamic assemblies formed at critical concentrations through cooperative, weak, multivalent interactions, often among disordered regions. These condensates activate genes by creating high local concentrations of active transcriptional components, enabling robust and precise changes in gene-regulatory programs. The PI’s recent work demonstrated that the formation of endogenous Myocardin (a potent cardiomyocyte and smooth muscle cell-specific transcriptional coactivator) condensates is a key molecular switch underlying cardiovascular lineage choice during development. This work unveiled a novel transcriptional regulatory mechanism governing cardiovascular lineage specification, and opened up new opportunities to understand how transcriptional condensates regulate cardiovascular cell fate transition. The biology of biomolecular condensate represents a flourishing research area with tremendous opportunities of making important findings. Our lab also aims to leverage the concept of transcriptional condensates to pioneer novel therapeutic strategies for cardiovascular diseases.
Relevant publications:
- Gan P, et al. Coactivator Condensation Drives Cardiovascular Cell Lineage Specification. Science Advances. 2024 Mar 15;10(11):eadk7160.
- Gan P, et al. Engineering a serum response factor superactivator of smooth muscle gene expression with a condensate-forming domain. PNAS. 2025 Sep 15.
About the PI

Peiheng Gan (甘沛恒), PhD, MBBS
Education and Experience:
07/2025-present: Assistant Professor, Temple University
08/2020-06/2025: Postdoctoral fellow, UTSW Medical Center
08/2015-05/2020: Ph.D., University of Southern California
09/2010-06/2015: MBBS, Shanghai Jiaotong University
Professional Affiliations:
2017-present: American Heart Association (AHA)
2023-present: AHA BCVS Early Career Committee
2022-present: Academy of Cardiovascular Research Excellence (ACRE)
2024-present: International Society for Heart Research (ISHR)
Selected Awards and Honors:
Shobha Ghosh Investigator in Training Award (Finalist), AHA, 2025
Paul Dudley White International Scholar Award, AHA, 2025
Pathway to Independence Award (K99/R00), NIH/NHLBI, 2024
Louis N. and Arnold M. Katz Basic Science Research Prize for Early Career Investigators (Finalist), AHA, 2023
Young Investigator Award (First Place), ACRE, 2023
BCVS New Investigator Travel Award, AHA, 2022
Inaugural CRSM Best Postdoc Presentation Award, UTSW, 2022
AHA postdoctoral fellowship, 2021-2023
AHA predoctoral fellowship, 2018-2020
National Undergraduate Innovative Research Award, Ministry of Education of China, 2012
Team (hiring now!)

Associate Scientist & Lab Manager
Hello, I am Hannah, I work best with mice but I can help you too. Targets: ensure protocols are up-to-date and followed, and supplies are available, accounted for, and are found instantly.

Postdoc Fellow
I’m a physician-scientist who spends a lot of time chasing data and occasionally chasing down coffee. The goal? Turn lab puzzles into bedside solutions.
Hiring:
- A postdoc fellow interested in transcriptional condensates in the cardiovascular system.
- 1-2 graduate students.
- Master/undergraduate students.
Publications
After independence: (2025-present):
- Tadokoro T, Li H, Gan P, Xu Z, Tan W, Alzhanov D, Sánchez-Ortiz E, McAnally J, Guo L, Xu L, Ruan P, Liu N, Olson EN. Ablation of PKCα Phosphorylation by CRISPR-Cas9 Base Editing Rescues Heart Failure. Circulation Research. 2026 Feb 20.
- Wang Z, Gan P*. Enhancer Dynamics for Gene Regulation in the Cardiovascular System. Arterioscler Thromb Vasc Biol. 2026 Jan 8. ( *corresponding author)
- Gan P*, Zhou X, Shah AM, Bezprozvannaya S, Chen K, Ding Q, Li H, Xu L, Liu N, Olson EN*. Engineering a serum response factor superactivator of smooth muscle gene expression with a condensate-forming domain. PNAS. 2025 Sep 15. ( *co-corresponding author)
Before independence (2015-2025):
- Caravia XM, Hayashi B, Li H, Gan P, Alzhanov D, Tan W, Chen K, McAnally J, Xu L, Liu N, Olson EN. Precise gene editing of pathogenic Lamin A mutations corrects cardiac disease. PNAS. 2025 Oct 13.
- Garry G, Dos Santos M, Chen K, Tan W, Bezprozvannaya S, Gan P, Xu L, Liu N, Bassel-Duby R, Olson EN. Cellular reprogramming by PHF7 enhances cardiac function following injury. Circulation. 2025 Jul 9.
- Ding Q, Gan P, Xu Z, Li H, Guo L, MacDonald C, Tan W, Sanchez-Ortiz E, McAnally J, Zhang Y, Karri D, Xu L, Liu N* & Olson EN*. Genomic Editing of a Pathogenic Mutation in ACTA2 Rescues Multisystemic Smooth Muscle Dysfunction Syndrome in Mice. Circulation. 2025 May 16. ( *co-corresponding author)
- Gan P#, Eppert M#, De La Cruz N, Lyons H, Shah AM, Veettil RT, Chen K, Pradhan, P, Bezprozvannaya S, Xu L, Liu N, Olson EN* & Sabari BR*. Coactivator Condensation Drives Cardiovascular Cell Lineage Specification. Science Advances. 2024 Mar 15;10(11):eadk7160. (#co-first author, *co-corresponding author)
- Gan P, Wang Z, Bezprozvannaya S, McAnally J, Tan W, Li H, Bassel-Duby R, Liu N, Olson EN. RBPMS Regulates Cardiomyocyte Contraction and Cardiac Function through RNA Alternative Splicing. Cardiovascular Research. 2024 Feb 27;120(1):56-68.
- Watanabe H, Tao G, Gan P, Westbury B, Cox K, Tjen K, Song R, Fishman G, Makita K, Sucov HM. Purkinje Cardiomyocytes of the Adult Ventricular Conduction System Are Highly Diploid but Not Uniquely Regenerative. J. Cardiovasc. Dev. Dis. 2023 April 7; 2023, 10(4), 161.
- Sodimu O, Almasian M, Gan P, Hassan S, Zhang X, Bassel-Duby R, Liu N, Ding Y. Light-Sheet Imaging and Interactive Analysis of the Cardiac Structure in Neonatal Mice. Journal of Biophotonics. 2023 Jan 9;e202200278.
- Caravia XM, Martinez AR, Gan P, Wang F, McAnally J, Xu L, Bassel-Duby R, Liu N, Olson EN. Loss of Function of the Nuclear Envelope Protein LEMD2 Causes DNA Damage-dependent Cardiomyopathy. Journal of Clinical Investigation. 2022;132(22):e158897.
- Gan P, Wang Z, Morales MG, Zhang Y, Bassel-Duby R, Liu N, Olson EN. RBPMS is an RNA-Binding Protein that Mediates Cardiomyocyte Binucleation and Cardiovascular Development. Developmental Cell. 2022 Apr 25;57(8):959-973.e7.
- Gan P, Baicu C, Watanabe H, Wang K, Tao G, Judge DP, Zile MR, Makita T, Mukherjee R, Sucov HM. The Prevalent I686T Human Variant and Loss of Function Mutations in the Cardiomyocyte-specific Kinase Gene TNNI3K Cause Adverse Contractility and Concentric Remodeling in Mice. Human Molecular Genetics. 2020 Oct 21:ddaa234.
- Gan P, Patterson M, Watanabe H, Wang K, Edmonds RA, Reinholdt LG, Sucov HM. Allelic Variants between Mouse Substrains BALB/cJ and BALB/cByJ Influence Mononuclear Cardiomyocyte Composition and Cardiomyocyte Nuclear Ploidy. Scientific Reports. 2020 May 5;10(1):7605.
- Shen H, Gan P, Wang K, Darehzereshki A, Wang K, Kumar SR, Lien CL, Patterson M, Tao G, Sucov HM. Mononuclear Diploid Cardiomyocytes Support Neonatal Mouse Heart Regeneration in Response to Paracrine IGF2 Signaling. Elife. 2020 Mar 13; 9: e53071.
- Gan P, Patterson M, Velasquez A, Wang K, Tian D, Windle J, Tao G, Judge D, Makita T, Park TJ, Sucov HM. Tnni3k Alleles Influence Ventricular Mononuclear Cardiomyocyte Frequency. PLoS Genetics. 2019 Oct 7;15(10): e1008354.
- Gan P, Patterson M, Sucov HM. Cardiomyocyte Polyploidy and Implications for Heart Regeneration. Annual Review of Physiology. 2019 Oct 4. Vol. 82. (Review)
- Wang K, Shen H, Gan P, Cavallero S, Kumar SR, Lien CL, Sucov HM. Differential Roles of Insulin Like Growth Factor 1 Receptor and Insulin Receptor during Embryonic Heart Development. BMC Developmental Biology. 2019 Mar 25;19(1):5.
- Patterson M, Barske L, van Handel B, Rau CD, Gan P, Sharma A, Parikh S, Denholtz M, Huang Y, Yamaguchi Y, Shen H, Allayee H, Crump JC, Force TI, Lien CL, Makita T, Lusis AJ, Kumar SR, Sucov HM. Frequency of Mononuclear Diploid Cardiomyocytes Underlies Natural Variation in Heart Regeneration. Nature Genetics. 2017 Sep;49(9):1346-1353.
News and Fun
Sep 27, 2025
Peiheng and Fei participated in the epic event to celebrate Dr. Eric Olson’s 70th birthday and 40-year lab reunion!


Sep 15, 2025
Peiheng’s first paper as a co-corresponding author was published on PNAS today!
Sep 04, 2025
With the arrival of Dr. Fei Luo, an outstanding cardiovascular physician-scientist, the lab is growing well!


Aug 04, 2025
Peiheng was interviewed for 2025 AHA PVD Council Communications.

Jul 01, 2025
The Gan lab is officially open!
May 16, 2025
Peiheng gave an oral presentation on 2025 Weinstein Cardiovascular Development and Regeneration Conference in Milwaukee, Wisconsin. The talk entitled “Mechanistic Insights into the Transcriptional Control of Cardiovascular Cell Fate by Condensates”.
April 25, 2025
2025 Vascular Discovery Scientific Sessions
Peiheng gave an oral presentation on transcriptional condensates and VSMC fate. This work was recognized by the Shobha Ghosh Investigator in Training Award competition and the Paul Dudley White International Scholar Award!
April 2, 2025